Did you know? The market for MicroRNA sequencing and assays, valued at USD 391.73 million in 2024, is projected to reach USD 1,369.11 million by 2034, growing at a 13.33% CAGR.
miRNAs have emerged as critical regulators of gene expression. Unlike traditional gene expression analysis, which focuses on protein-coding genes, miRNA sequencing uncovers the regulatory networks that control cellular behavior.
This proves invaluable for drug discovery, where understanding regulatory mechanisms can reveal new therapeutic targets and predict drug responses. For companies developing precision medicine approaches, miRNA profiles offer patient stratification opportunities that complement genomic data.
In this article, we’ll explore the complete miRNA sequencing workflow, from sample collection through data interpretation, while addressing the unique challenges these small RNA molecules present.
Key Takeaways
- MicroRNA sequencing involves specialized workflows designed to capture and analyze small RNA molecules that regulate gene expression.
- Key steps include careful sample preparation with RNA integrity preservation, adapter ligation-based library preparation, high-throughput sequencing on platforms like Illumina, and computational analysis for quantification and target prediction.
- Primary applications include biomarker discovery, drug target identification, and therapeutic monitoring in pharmaceutical development.
- Major challenges include size-selection bias, contamination from other small RNAs, and the need for specialized bioinformatics expertise.
- Advanced AI-driven platforms like Biostate AI are addressing these challenges by providing end-to-end solutions with automated workflows, reducing costs from hundreds to $80 per sample while maintaining high accuracy.
What is MicroRNA Sequencing?
MicroRNAs (miRNAs) are a class of small, single-stranded, non-coding RNA molecules, typically ranging from 18 to 25 nucleotides in length. They exert their primary biological function in post-transcriptional gene regulation by binding to complementary sequences on messenger RNAs (mRNAs).
MicroRNA sequencing (miRNA-seq) represents a specialized form of RNA sequencing designed specifically to detect, quantify, and characterize small RNA molecules, particularly microRNAs.
Unlike standard RNA sequencing that captures the full spectrum of cellular transcripts, miRNA-seq employs targeted approaches to enrich for small RNA species while excluding longer transcripts that would otherwise dominate the sequencing libraries.
Key Principle
The fundamental principle behind miRNA-seq exploits the unique structural characteristics of microRNAs.
- These molecules undergo specific processing steps in cells, from initial pri-miRNA transcripts to mature miRNAs, each step creating distinct molecular signatures.
- The sequencing process captures these mature forms, typically 20-24 nucleotides long, providing quantitative measurements of miRNA expression levels across different conditions, tissues, or time points.
- What distinguishes miRNA-seq from other sequencing approaches is its focus on regulatory elements rather than protein-coding sequences.
While messenger RNA sequencing reveals what proteins cells are preparing to make, miRNA sequencing uncovers the regulatory instructions that determine when, where, and how much protein actually gets produced.
Now let’s see the workflow of miRNA sequencing.
The Workflow for MicroRNA Sequencing
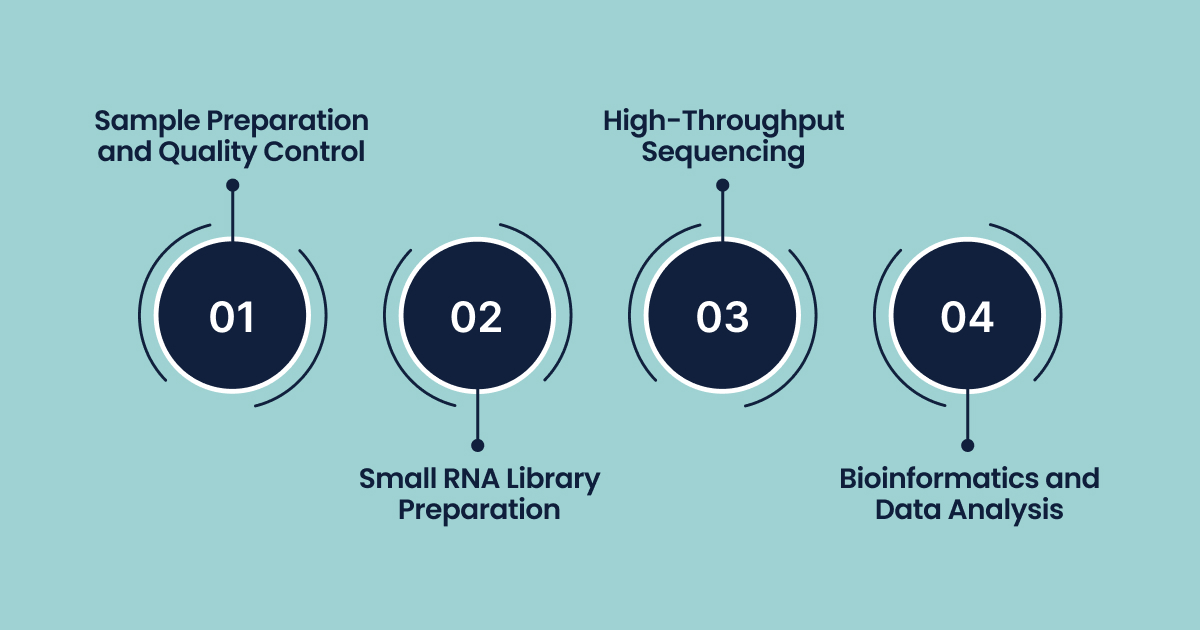
The miRNA sequencing process is multi-faceted, requiring precision at every step to ensure high-quality data generation.
Step 1: Sample Preparation and Quality Control
The first and most crucial step in miRNA sequencing is selecting the right biological sample, which can range from tissues and cell cultures to more complex biofluids like blood plasma.
- miRNAs, especially from formalin-fixed paraffin-embedded (FFPE) tissues, are highly stable, making them easier to extract compared to other RNA species.
- Optimizing extraction methods like TRIzol or column-based kits is essential to efficiently capture the small RNA fraction.
- For blood plasma, RNase activity is a key challenge, requiring rapid enzyme inactivation to prevent miRNA degradation.
- In tissue samples, cellular heterogeneity must be addressed, as miRNAs often have tissue-specific expression.
- Techniques like microdissection can help target specific cell types, ensuring miRNAs are representative of the desired tissue.
High-quality RNA extraction directly impacts the reliability of miRNA sequencing and the clinical relevance of the data.
- After extraction, assessing RNA quality and quantity is critical. Spectrophotometric methods (UV absorbance) and electrophoresis (e.g., Agilent Bioanalyzer) are standard.
Maintaining RNA integrity is crucial for reliable sequencing, and stringent protocols must be followed to mitigate pre-analytical variability, especially in large-scale studies or clinical trials.
Step 2: Small RNA Library Preparation
Small RNA library preparation is a crucial step in miRNA sequencing. This is where miRNAs are enriched from the total RNA pool using size selection techniques like gel electrophoresis or size-exclusion chromatography.
- Once enriched, specialized adapters are ligated to both the 3′ and 5′ ends of the small RNA molecules. The 3′ adapter is often modified to target miRNAs with a 3′ hydroxyl group, a feature of enzymatic cleavage.
- To prevent adapter dimers, advanced kits like NEBNext use reverse transcription primers that hybridize to excess 3′ adapters, preventing unwanted ligation of 5′ adapters.
- Alternative protocols, such as RealSeq®-AC, use single adapter ligation with circularization to enhance ligation efficiency and reduce biases.
Reverse transcription is then carried out using stem-looped primers or poly(A) tails to ensure efficient conversion of miRNAs into cDNA.
Following reverse transcription, Polymerase Chain Reaction (PCR) amplification is performed to exponentially increase the cDNA library.
- Optimizing PCR conditions is critical to minimize amplification bias and artifacts, ensuring accurate reflection of miRNA abundance.
- To further enhance specificity, masking oligonucleotides targeting abundant rRNAs (e.g., 5.8s rRNA) can be included.
After amplification, the libraries are evaluated for quality and quantity using capillary electrophoresis or fluorometry.
- Libraries are then normalized and pooled for sequencing.
A key advancement in library preparation is the use of unique barcodes. Unique barcodes are short DNA sequences added to each library sample, allowing multiple samples to be pooled and sequenced together (multiplexing), which increases throughput and reduces costs
However, technical biases introduced during amplification, such as adapter choice, can affect miRNA quantification, requiring careful kit selection and bioinformatics normalization to ensure accurate data.
Step 3: High-Throughput Sequencing
For miRNA sequencing, the meticulously prepared cDNA libraries are loaded onto high-throughput NGS platforms.
- On these platforms, the sequencing-by-synthesis (SBS) principle is typically employed, where fluorescently labeled nucleotides are sequentially incorporated into growing complementary DNA strands.
- This process generates millions of short sequence reads that collectively represent the comprehensive small RNA composition of the original biological sample.
The “sequencing depth,” which refers to the total number of reads generated per sample, is a critical parameter that must be carefully tailored to achieve the desired coverage and sensitivity necessary for precise and reliable miRNA expression analysis
Step 4: Bioinformatics and Data Analysis
The bioinformatics and data analysis process for miRNA sequencing involves several steps:
Preprocessing
- Raw sequencing data undergoes removal of adapters, low-quality reads, and artifacts to ensure reliable downstream analysis.
- Specialized tools like Cutadapt and Trimmomatic are used due to the short length of miRNA reads (18-30 nucleotides).
- Stringent quality control, including sequence quality scores and GC content distribution, is essential for maintaining data integrity.
Mapping and Alignment
- Mapping short miRNA reads presents challenges, especially due to multi-mapping (reads aligning to multiple genome locations).
- Tools like Bowtie2 and the STAR aligner are optimized for small RNA data and used with fine-tuned parameters for better accuracy.
- miRDeep2 aids in miRNA annotation, distinguishing them from other small RNA species like tRNA fragments.
Quantification and IsomiR Detection
- Accurate miRNA quantification requires distinguishing closely related miRNA family members and variants (isomiRs).
- Tools like isomiRage and seqBuster provide a more detailed view of the miRNA landscape by identifying and quantifying isomiRs.
Normalization:
- Traditional normalization methods (e.g., RPM) may fail to address compositional biases in small RNA sequencing data.
- Absolute normalization, including spike-in controls, ensures accurate quantitative comparisons across different sample types and experimental groups.
Bioinformatics Expertise and Tools:
- miRNA data analysis is complex and requires specialized bioinformatics pipelines, including preprocessing, alignment, and annotation tools.
- Platforms like Illumina’s BaseSpace Sequence Hub offer user-friendly applications for differential expression analysis, 3P/5P miRNA ratio analysis, and more.
- Publicly available tools like the ENCODE miRNA-seq pipeline offer standardized methods for small RNA processing.
Modern miRNA-seq platforms can process hundreds of samples simultaneously, making large-scale studies feasible for pharmaceutical companies investigating patient cohorts or conducting drug response studies. Let’s see about it in the next section.
Sequencing Process and Platforms
The sequencing phase of miRNA analysis requires platforms optimized for short-read sequencing with high accuracy and sufficient depth to detect low-abundance miRNAs. Platform selection significantly impacts data quality, cost, and the types of analysis possible with the resulting data.
Illumina Systems: High-Throughput and Precision
Illumina platforms operate on the principle of sequencing-by-synthesis (SBS) technology, enabling the simultaneous, massively parallel sequencing of millions to billions of nucleic acid fragments. This core technology allows for comprehensive and precise miRNA profiling
- MiSeq: Ideal for small-scale projects with 1-25 million reads and read lengths up to 2×300 bp. Suitable for targeted sequencing and miRNA analysis.
- NextSeq: Offers up to 1.8 billion reads and 540 Gb output, optimized for miRNA-Seq and other RNA applications. Flexible and scalable for mid- to large-scale projects.
- NovaSeq: High-throughput production sequencer with up to 8 Tb per flow cell and 52 billion paired-end reads. Perfect for large-scale sequencing and miRNA profiling with deep coverage.
Thermo Fisher Scientific (Ion Torrent): Speed and Simplicity
Thermo Fisher Scientific’s Ion Torrent platforms utilize semiconductor-based sequencing technology that detects changes in pH caused by nucleotide incorporation. This method provides a fast and simple workflow, making it attractive for various RNA sequencing applications, including small RNA and miRNA sequencing
- Ion GeneStudio S5: Provides rapid sequencing with outputs of 15-50 Gb per day. Ideal for targeted gene expression analysis, particularly in challenging samples like FFPE tissues.
- Limitations: Higher error rates in homopolymer regions may impact indel detection, but it still offers excellent reproducibility and variant calling accuracy.
Pacific Biosciences (PacBio): Long Reads for Isoform and Small RNA Characterization
Pacific Biosciences (PacBio) offers Single-Molecule Real-Time (SMRT) sequencing technology, which is renowned for generating exceptionally long reads, often exceeding 10 kb.
While traditionally applied to full-length transcript sequencing (Iso-Seq) for comprehensive isoform characterization, its relevance for miRNA sequencing, which involves much shorter molecules, requires specific consideration.
- HiFi Reads: High-accuracy reads with less than 1% error rate. While not the primary choice for miRNA sequencing, PacBio can help explore complex small RNA species and their precursors.
- While the primary focus of PacBio is on long reads, some service providers like Psomagen do offer small RNA sequencing services. This indicates potential adaptations or specific niche applications where long-read capabilities might still be leveraged for small RNA analysis.
The ability of PacBio to sequence full-length cDNAs derived from small RNAs, if specific library preparation strategies are employed, could offer advantages in characterizing complex small RNA species or their precursors, which might extend beyond the typical mature miRNA length.
Oxford Nanopore Technologies (ONT): Portability and Real-Time Data
ONT offers the ability to sequence native DNA or RNA without amplification. This direct sequencing capability (e.g., SQK-RNA004) can avoid biases introduced by PCR amplification steps. ONT devices can produce reads of any length, from short (50 bp) to ultra-long (over 4 Mb)
- MinION: Portable sequencer providing up to 50 Gb per flow cell, ideal for on-site and rapid sequencing with real-time analysis.
- GridION: A Scalable system running up to 5 MinION flow cells, ideal for multi-project sequencing.
- PromethION: High-throughput platform with up to 290 Gb per flow cell, suited for large-scale studies.
However, a key challenge for ONT with very short reads, like miRNAs, has been lower accuracy compared to sequencing-by-synthesis methods, especially for sequences shorter than 100 bp.
While ONT has improved accuracy through algorithm updates and hardware advancements (e.g., R10 chip) and consensus sequencing (multiple passes), achieving high accuracy for short, low-abundance miRNAs remains a limitation.
Now, let’s see the role of miRNA sequencing in therapeutic research.
Applications of MicroRNA Sequencing
miRNA-seq offers several advantages over traditional expression analysis. The regulatory nature of miRNAs means they often change earlier in disease progression than protein-coding genes, potentially providing earlier diagnostic markers.
Additionally, miRNAs can be detected in various biological fluids, including blood, urine, and saliva, making them accessible biomarkers for non-invasive monitoring applications.
Biomarker Discovery and Validation
MiRNAs have emerged as a highly promising class of biomolecules for diagnostic biomarker applications due to their remarkable stability in various biological fluids and their disease-specific expression patterns.
- Early Disease Detection: miRNAs’ stability in liquid biopsies (serum, plasma) allows for non-invasive early disease detection and monitoring. For instance, specific miRNAs in non-small-cell lung cancer (NSCLC) have been identified as early biomarkers, providing insights into disease onset and progression.
- Prognosis and Disease Monitoring: miRNAs offer valuable prognostic information, such as miR-122 for liver injury and miR-133a for muscle damage. This enables better patient management and tracking treatment responses in various conditions, including cancer and cardiovascular diseases.
- Patient Stratification and Companion Diagnostics: miRNA signatures help in tailoring treatments by identifying molecular profiles, enhancing patient stratification, and supporting precision medicine.
Companies like Biostate AI are integrating AI to refine diagnostic solutions in diseases like cancer and cardiovascular disorders.
Drug Discovery and Development
- Target Identification and Validation: miRNA sequencing aids in discovering disease mechanisms and potential drug targets. Companies like Mirna Therapeutics are developing miRNA-targeted therapies, such as MRX34, for liver cancer.
- Drug Efficacy and Toxicity: miRNA profiles help assess drug-induced toxicity, offering early safety biomarkers for preclinical and clinical phases. miRNAs also play a role in chemotherapy resistance, offering insights into cancer treatment responses.
- Therapeutic Development: miRNAs are being explored as therapeutic agents. Companies like miRagen Therapeutics are developing miRNA mimics and inhibitors, such as MRG-106, to regulate disease-related miRNAs for therapeutic benefits.
Advancements in Single-Cell and Spatial Profiling
The field of miRNA sequencing is continually evolving, with single-cell and spatial profiling technologies opening new avenues for understanding disease mechanisms.
- Single-Cell miRNA Sequencing: This approach reveals cellular diversity at the individual cell level, helping identify rare cell types or gene expression variations that influence disease progression, drug resistance, and tumor relapse.
- Spatial miRNA Profiling: Spatial transcriptomics enhances gene expression insights by contextualizing miRNAs in their original tissue architecture.
Technologies, like NanoString GeoMx® Digital Spatial Profiler and CosMx™ Spatial Molecular Imager, enable high-plex, spatial molecular imaging, offering a deeper understanding of cellular interactions and disease mechanisms.
Despite significant technological advances, miRNA sequencing continues to face substantial challenges that impact data quality, reproducibility, and clinical translation. Let’s find out.
Challenges in MicroRNA Sequencing
miRNA bioinformatics is complex, involving several technical challenges. Generating raw sequencing data is not enough. Transforming this data into actionable insights relies on expert-driven bioinformatic pipelines.
- Short Read Multi-Mapping
miRNA reads often map to multiple genome locations, causing ambiguity and complicating target identification.
- IsomiR Detection
miRNAs can exist as isomiRs, variants that differ slightly in sequence or length. Detecting and quantifying these requires specialized tools, adding complexity to the analysis.
- Normalization Issues
Traditional normalization methods like Reads Per Million (RPM) may not account for biases in small RNA data, leading to inaccurate comparisons. More advanced strategies, including absolute normalization and spike-in controls, are necessary for reliable results.
- Bioinformatics Complexity
Properly processing miRNA data requires robust pipelines for preprocessing (e.g., Cutadapt), alignment (Bowtie2, STAR), and annotation (miRDeep2). This complexity can create bottlenecks, especially without specialized computational resources.
- Reliability and Reproducibility
Variability introduced during sample preparation, sequencing, and analysis can affect the reliability and reproducibility of results. Addressing these inconsistencies is critical to achieving robust, reproducible findings, especially for large-scale or multi-center studies.
These challenges highlight the importance of specialized bioinformatics expertise and tools in miRNA sequencing.
Service providers like Biostate AI offer comprehensive analysis solutions, including data interpretation and normalization, to support pharmaceutical and biotechnology companies in leveraging miRNA data effectively.
How Biostate AI is Transforming RNA-Sequencing Technology
Biostate AI represents a paradigm shift in RNA sequencing technology, addressing the fundamental challenges that have limited the accessibility and clinical utility of miRNA sequencing. By combining artificial intelligence and streamlined workflows, we democratize access to high-quality RNA sequencing while dramatically reducing costs and complexity.
Here’s what we offer:
- Unbeatable Pricing: High-quality sequencing results starting at $80 per sample, making large-scale studies financially accessible without compromising data quality or analytical depth.
- Rapid Turnaround: Complete results delivered in just 1-3 weeks, accelerating research timelines and enabling faster progression from hypothesis to publication.
- Complete Transcriptome Coverage: Comprehensive RNA-Seq analysis covering both mRNA and non-coding RNA species, providing holistic insights into gene expression and regulatory networks.
- AI-Driven Analysis: Access to the OmicsWeb AI platform that transforms raw sequencing data into intuitive, publication-ready insights without requiring extensive bioinformatics expertise.
- Minimal Sample Requirements: Advanced protocols that can process samples as small as 10µL blood, 10ng RNA, or 1 FFPE slide, maximizing the utility of precious clinical samples.
- Low RIN Compatibility: Specialized chemistry that works with degraded RNA samples having RIN values as low as 2, compared to the typical requirement of RIN ≥5 for standard protocols.
This integrated approach transforms RNA sequencing from a complex technical challenge into a streamlined research tool that delivers reliable, actionable results while maintaining the highest standards of scientific rigor.
Conclusion
As the technology continues to evolve, the integration of miRNA sequencing with other omics technologies will create comprehensive platforms for understanding disease mechanisms and optimizing therapeutic approaches.
Historically, challenges such as high costs, technical complexity, and the need for specialized expertise have limited miRNA sequencing adoption.
The question for you is not whether to adopt these technologies, but how quickly you can integrate them into your development programs to gain competitive advantages.
Biostate AI offers RNA-seq services starting from $80 per sample. Whether you are conducting longitudinal biomarker studies or acute drug response experiments, our multi-omics support, two-stage AI framework, and automated analysis pipeline routine can potentially reduce the time and cost required for your research.
Get in touch with us for tailored solutions to your specific research needs.
FAQs
Q1: What is the typical turnaround time and cost for miRNA sequencing projects?
Traditional miRNA sequencing costs $300-500 per sample with 4-8 week turnaround times. Advanced platforms like Biostate AI deliver results in 1-3 weeks at $80 per sample, making large-scale biomarker studies economically viable for pharmaceutical companies.
Q2: How does miRNA sequencing differ from standard RNA-seq?
Standard RNA-seq reveals what proteins cells prepare to make, while miRNA sequencing shows the regulatory instructions controlling protein production. miRNAs change earlier in disease progression, can be detected in blood/urine for non-invasive testing, and remain stable in challenging clinical samples where standard RNA-seq would fail.
Q3: What are the key technical challenges in miRNA sequencing?
Main challenges include adapter ligation bias, size selection difficulties, and specialized bioinformatics requirements. Platforms like Biostate AI address these through optimized protocols, randomized adapters, and AI-driven analysis pipelines that eliminate the need for specialized computational expertise.
Q4: How can miRNA sequencing integrate into drug development workflows?
miRNA sequencing works across all development stages: target identification in discovery, toxicity prediction in optimization, and patient stratification in clinical trials. Start with focused pilot studies, then expand as expertise develops. End-to-end platforms simplify integration by eliminating multiple vendor coordination.
