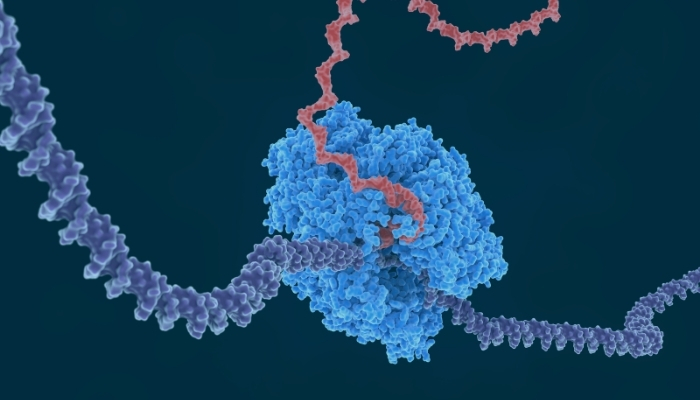
Messenger RNA (mRNA) synthesis is a fundamental biological process that enables cells to convert genetic instructions encoded in DNA into functional proteins. Acting as a critical intermediary, mRNA carries the genetic blueprint from the nucleus to the ribosome, where proteins are assembled, making it central to virtually every cellular function.
The clinical importance of mRNA became widely recognized during the COVID-19 pandemic, when mRNA vaccines showcased the power of manipulating this process for therapeutic benefit. Since then, mRNA-based therapies have gained momentum in treating cancer, genetic disorders, and other chronic diseases.
In this article, we’ll explore the whole journey of mRNA from its transcription and protective modifications to its role in protein translation and export, highlighting why this process is vital for both cellular health and modern medicine.
TL;DR
mRNA synthesis is the process by which cells create messenger RNA from DNA, bridging genetic information to protein production.
- It involves transcription (initiation, elongation, and termination), RNA processing (capping, splicing, and polyadenylation), and mRNA export for translation.
- Advances in mRNA technology have led to successful applications in vaccines (e.g., COVID-19), cancer therapy, and rare diseases by delivering functional proteins or correcting genetic mutations.
- Despite challenges like RNA degradation and incomplete processing, innovations such as lipid nanoparticles for delivery and next-gen sequencing methods are transforming therapeutic approaches.
What is mRNA Synthesis?
Messenger RNA synthesis, also known as transcription, transforms genetic blueprints into transportable molecular messages that carry instructions from the nucleus to the protein-manufacturing machinery in the cytoplasm.
- The mRNA synthesis process begins when RNA polymerase II recognizes specific promoter sequences upstream of target genes, initiating a carefully orchestrated series of molecular events.
- The synthesis process involves three distinct phases: initiation, elongation, and termination.
- During initiation, transcription factors assemble at the promoter region, creating a pre-initiation complex that positions RNA polymerase II at the transcription start site.
- Recent advances in cryo-electron microscopy have revealed unprecedented details about this complex assembly, with companies like the Structural Genomics Consortium utilizing these insights to develop more precise therapeutic targets.
Now that we understand what mRNA synthesis entails, it’s essential to examine the step-by-step process, known as transcription, that converts DNA blueprints into pre-mRNA molecules ready for further refinement.
The Transcriptional Process: From DNA to Pre-mRNA
The transcription process is divided into three primary steps: initiation, elongation, and termination. Here’s how the process unfolds:
Transcription Initiation: Starting the Process
Transcription begins when RNA polymerase II, the enzyme responsible for mRNA synthesis, recognizes specific DNA sequences known as promoters.
- These regions contain binding sites for transcription factors, which help position the polymerase at the correct starting point.
- The formation of the transcription initiation complex is a multistep process.
- General transcription factors bind to the promoter in a precise order, forming a stable platform that recruits RNA polymerase II. This ensures that transcription starts at the correct location and moves in the proper direction.
- Interestingly, enhancer sequences, even if located far from the gene, can influence initiation via DNA looping.
- These three-dimensional interactions enable distant regulatory elements to physically interact with promoters, adding another layer of gene expression control.
- Recent structural biology studies have shed light on the conformational changes RNA polymerase II undergoes during initiation.
- The enzyme transitions from a closed complex to an open complex, unwinding the DNA helix to form a transcriptional bubble.
Companies like Genentech are now leveraging this mechanistic insight to develop small-molecule inhibitors that selectively disrupt transcription in cancer cells.
Transcription Elongation: Building the mRNA
Once transcription is initiated, RNA polymerase II traverses the DNA template, adding complementary nucleotides to the growing pre-mRNA strand.
- This phase, however, isn’t without obstacles. Nucleosomes, DNA-binding proteins, and regulatory elements can hinder polymerase progression.
- Here, elongation factors step in. For example, P-TEFb phosphorylates RNA polymerase II, enhancing its processivity and reducing premature termination.
- Without such regulatory proteins, many genes would fail to produce full-length mRNA.
- The rate of elongation varies depending on the gene and cell type. Some genes take minutes to transcribe; others require hours.
- This variability contributes to the fine-tuned regulation of gene expression across different tissues.
- Importantly, RNA processing often begins during transcription.
This co-transcriptional modification, including 5′ capping and intron splicing, enhances efficiency and ensures the production of functional mRNA.
Transcription Termination: Ending the Process
Termination occurs when RNA polymerase II encounters specific termination signals in the DNA, prompting it to halt mRNA synthesis and disengage from the DNA template.
- Accurate termination is critical to ensure that the mRNA transcript is complete and properly processed.
- One key termination signal is the polyadenylation sequence, located near the 3′ end of genes.
- This sequence triggers a complex cascade: cleavage of the pre-mRNA, addition of a poly(A) tail, and release of RNA polymerase. This event simultaneously marks the end of transcription and the beginning of 3′ end processing.
- Failures in transcription termination can lead to read-through transcription, where RNA polymerase ignores the stop signal and continues into downstream genes.
- This misregulation can interfere with neighboring gene expression and has been linked to various diseases.
While transcription produces the initial RNA transcript, this molecule is not yet ready for its cellular role. It must undergo a series of vital processing events to become a mature, functional mRNA.
Essential RNA Processing Events During mRNA Synthesis
Co- and post-transcriptional modifications, 5′ capping, splicing, and 3′ polyadenylation, are essential for mRNA stability, nuclear export, and efficient translation.
Among the first and most crucial of these modifications is the addition of a protective cap to the 5’ end of the mRNA, a process that ensures the molecule’s stability and future utility.
5′ Capping: Protecting the Message
Within seconds of transcription initiation, the 5′ end of nascent pre-mRNA is modified with a 7-methylguanosine cap. This cap is added via three enzymatic steps while RNA polymerase II still elongates the transcript.
- The cap structure:
- Shields mRNA from exonuclease degradation
- Enhances ribosome binding for translation
- Facilitates nuclear export via cap-binding proteins
Capping enzymes are recruited through interactions with the phosphorylated C-terminal domain (CTD) of RNA polymerase II, ensuring that capping occurs co-transcriptionally.
Pharmaceutical companies like Moderna and BioNTech optimize cap analogs in mRNA therapeutics to improve transcript stability and reduce innate immune activation. However, achieving uniform capping efficiency across various sequences remains an active area of research.
Splicing: Removing Unnecessary Sequences
Most eukaryotic genes contain introns that interrupt coding regions (exons). During splicing, the spliceosome, a dynamic ribonucleoprotein complex, removes introns and joins exons to form a continuous coding sequence.
- Accurate splicing is vital because even small errors can lead to frameshifts or premature stop codons.
- Around 15% of known pathogenic mutations impact splicing patterns.
- Alternative splicing occurs in over 90% of human genes, enabling tissue-specific isoforms and proteomic diversity.
Platforms like Biostate AI leverage long-read sequencing to resolve full-length transcript isoforms and distinguish between spliced and unspliced RNA, providing high-resolution insights into transcript structure across biological conditions. Similarly, Pacific Biosciences technologies capture splice variants often missed by short-read sequencing.
3′ End Processing: Completing the Message
At the transcript’s 3′ end, cleavage and polyadenylation complete the maturation process.
- CPSF (Cleavage and Polyadenylation Specificity Factor) recognizes the canonical AAUAAA sequence
- Additional proteins bind U/GU-rich downstream elements, guiding endonucleolytic cleavage
- Poly(A) polymerase adds a tail of ~200 adenine residues post-cleavage
This poly(A) tail:
- Stabilizes mRNA by protecting against degradation
- Promotes translation via interaction with PABPs (Poly(A)-Binding Proteins)
Deadenylation marks the first step in mRNA decay, and tail length can change dynamically during stress, development, or disease. Novel techniques like poly(A) tail-length profiling now allow researchers to study these shifts as biomarkers for RNA stability and translational control.
Once all essential modifications are complete, the mature mRNA must pass stringent quality control checks before it can exit the nucleus and proceed to its next destination.
Nuclear Export and Quality Control
Mature mRNA molecules must exit the nucleus to reach cytoplasmic ribosomes for translation.
Export Requirements and Mechanisms
Nuclear export requires multiple quality control checkpoints that ensure only properly processed mRNA molecules leave the nucleus.
- Export factors such as NXF1 (nuclear RNA export factor 1) and ALYREF recognize specific features of mature mRNA, including the 5′ cap, properly spliced exon junctions, and the 3′ poly(A) tail. These features help distinguish export-competent transcripts from improperly processed ones.
- The nuclear pore complex (NPC) acts as both a gate and a quality control checkpoint. Only transcripts properly assembled into messenger ribonucleoprotein particles (mRNPs) can pass through.
- The exon junction complex (EJC), deposited during splicing ~20–24 nucleotides upstream of exon–exon junctions, serves as a signal for downstream processing and export. Its presence helps facilitate the recognition of mature mRNA by export receptors.
- Defective or incompletely processed transcripts are retained in the nucleus and targeted for degradation by surveillance mechanisms, including the nuclear exosome and TRAMP complex.
- This prevents the production of aberrant proteins and maintains transcriptome integrity.
Cytoplasmic Fate Determination
After successfully navigating nuclear export, mRNA molecules encounter a new set of regulatory influences in the cytoplasm that dictate their stability, localization, and eventual translation into protein.
- In the cytoplasm, RNA-binding proteins (RBPs) such as HuR, TTP, and PABP bind to sequence elements in mRNA and regulate localization, stability, or translation.
- MicroRNAs (miRNAs), in complex with the RNA-induced silencing complex (RISC), bind to complementary sequences in the 3′ UTRs of target mRNAs. This typically results in translational repression or degradation, allowing for fine-tuned post-transcriptional control.
- Under stress conditions, mRNAs and associated proteins are sequestered into stress granules, while degraded transcripts are often directed to processing bodies (P-bodies).
- The dynamic movement between these compartments determines whether an mRNA will be stored, re-enter translation, or be degraded.
- Advanced methods such as CLIP-seq (crosslinking and immunoprecipitation sequencing) and Ribo-seq (ribosome profiling) now allow researchers to map RBP binding and translation status of mRNAs at scale, revealing how cytoplasmic regulation shapes gene expression in real time.
With its fate determined, the mRNA’s ultimate purpose is realized as it serves as a template for protein synthesis—a process known as translation that brings genetic instructions to life.
Translation: From mRNA to Protein
Translation begins when ribosomes recognize and bind to mRNA molecules through interactions with the 5′ cap and scanning for the AUG start codon downstream. This process requires multiple initiation factors, such as eIF4E, eIF2, and the eIF4F complex, that help position ribosomes at the correct start codon.
Ribosome Recruitment and Initiation
The 5′ untranslated region of mRNA contains regulatory elements that influence translation efficiency.
- Secondary structures, upstream open reading frames, and protein binding sites can enhance or inhibit ribosome recruitment, providing additional layers of gene expression control.
- Translation initiation represents a major control point where cells can rapidly adjust protein synthesis rates.
- Growth factors, hormones, and stress conditions often target translation initiation rather than transcription, allowing faster responses to environmental changes.
- Ribosome profiling techniques reveal the dynamics of translation initiation across different mRNA molecules.
These approaches show that ribosome occupancy varies significantly among genes and changes in response to cellular conditions.
Elongation and Protein Synthesis
Ribosomal elongation proceeds through coordinated cycles of amino acid addition to the growing protein chain.
- Transfer RNAs deliver specific amino acids to ribosomes based on codon-anticodon interactions, ensuring accurate protein synthesis.
- Elongation rate varies among different codons and can be influenced by tRNA availability and mRNA secondary structures.
- Slow elongation at certain codons may facilitate proper protein folding or provide opportunities for co-translational protein modifications.
- Quality control mechanisms during elongation prevent the incorporation of incorrect amino acids and deal with ribosomes that stall on damaged mRNA molecules.
- These surveillance systems maintain protein synthesis fidelity while allowing recovery from translation problems.
- The genetic code’s redundancy provides some protection against mutations, as many nucleotide changes do not alter the amino acid sequence.
- However, mutations that change amino acids or create premature stop codons can have severe consequences for protein function.
As our understanding of mRNA translation deepens, innovative technologies are rapidly emerging to analyze, manipulate, and harness these processes for therapeutic and research applications.
Technological Advances in mRNA Analysis
Recent advancements in mRNA technology have revolutionized therapeutic development, particularly in delivery systems, gene editing, and clinical applications. These innovations address historical challenges in stability, delivery efficiency, and targeted protein expression.
- Delivery System Innovations
Lipid Nanoparticles (LNPs) remain the gold standard, with microfluidics enabling precise manufacturing. This technology mixes mRNA with lipid components at controlled ratios, producing uniform particles (~80–120 nm) that enhance cellular uptake.
New formulations like proteolipid vehicles (PLVs) combine fusogenic proteins with lipids, enabling direct cytoplasmic delivery without endosomal escape and accommodating larger payloads like plasmid DNA.
Targeted Delivery advancements include:
- Liver-focused LNPs using ionizable lipids with pKa ~6.5
- Lung-targeted systems via inhalation (e.g., Translate Bio’s CFTR mRNA trial)
- Tunable surface chemistries for spleen or tumor accumulation.
- Next-Generation Sequencing Approaches
Single-cell approaches reveal transcriptional heterogeneity within cell populations, while spatial methods maintain tissue context.
- Long-read sequencing platforms enable comprehensive characterization of full-length transcripts, revealing alternative splicing patterns and RNA modifications that short-read methods miss.
- Oxford Nanopore Technologies has demonstrated direct RNA sequencing capabilities that preserve native base modifications like m6A, which are lost in traditional reverse-transcription workflows.
- Portable sequencing devices open new possibilities for point-of-care diagnostics and field-based research.
However, current limitations include higher error rates and reduced throughput compared to established laboratory platforms.
Biostate AI’sOmicsWeb platform integrates multiple sequencing modalities—short-read, long-read, single-cell, and spatial—into a single, integrated ecosystem. Whether profiling whole transcriptomes or investigating isoform diversity, OmicsWeb enables you to analyze transcriptional landscapes across various resolution scales easily.
Its built-in AI copilot transforms data exploration by letting you ask complex biological questions in plain English; no coding or command-line tools required.
Clinical Applications and Therapeutic Implications of mRNA Synthesis
The success of mRNA vaccines has demonstrated the therapeutic potential of synthetic mRNA molecules. These vaccines work by delivering mRNA encoding viral proteins to cells, which then produce the proteins and stimulate immune responses.
- mRNA Vaccines in Infectious Diseases
mRNA vaccines have demonstrated remarkable success in combating viral diseases, with the COVID-19 pandemic accelerating their development. Notable examples include:
- COVID-19: The Pfizer/BioNTech and Moderna vaccines were among the first mRNA vaccines to receive approval. These vaccines encode the spike (S) protein of SARS-CoV-2, enabling host cells to produce the antigen and initiate an adaptive immune response. They also showed high efficacy rates (95% and 94.1%, respectively). By stabilizing the spike protein in its pre-fusion form, these vaccines trigger a strong immune response, making them highly effective in preventing COVID-19.
- Respiratory Syncytial Virus (RSV): Moderna’s mRNA-1345 vaccine has demonstrated an 83.7% efficacy rate in preventing RSV in adults aged 60 and above. By encoding the RSV F glycoprotein, the vaccine elicits a strong immune response, with approval from the FDA in 2024.
- Influenza: The mRNA-1010 vaccine, currently under development, targets four influenza strains. This vaccine is highly adaptable and can rapidly be updated to respond to new viral strains, providing an advantage over traditional flu vaccines.
- Cytomegalovirus (CMV): The mRNA-1647 vaccine, under investigation in clinical trials, aims to combat CMV, which affects immunocompromised individuals and newborns. This vaccine stimulates both cellular and humoral immune responses to offer protection.
2. mRNA in Cancer Therapy
mRNA vaccines are emerging as powerful tools in cancer immunotherapy, aiming to train the immune system to recognize and destroy tumor cells:
- Tumor-Specific Antigens (TSAs) and Tumor-Associated Antigens (TAAs): mRNA vaccines can be engineered to encode specific tumor antigens, stimulating the immune system to target cancer cells. For example, the BNT111 mRNA vaccine targets four TAAs, showing promising results in melanoma patients.
- Combination Therapies: Combining mRNA vaccines with immune checkpoint inhibitors or other therapies enhances the immune response against tumors. The mRNA-4157/V940 vaccine, combined with pembrolizumab, reduced recurrence and mortality rates in melanoma patients, showcasing the potential of mRNA-based therapies in cancer.
- Personalized Cancer Vaccines: Personalized mRNA vaccines represent a promising frontier in oncology by tailoring immunotherapy to a patient’s unique tumor profile.Emerging platforms, such as tumor-specific open reading frame (ORF) discovery pipelines, allow researchers to identify patient-specific neoantigens and encode them into customized mRNA vaccines. While acronyms like TOFU (Tumor-specific Open Reading Frame) have been used informally in exploratory projects or internal research pipelines, they are not standardized terms in published literature.
3. mRNA Therapies for Protein Deficiency Diseases and Rare Diseases
mRNA therapies offer a promising solution for treating rare diseases caused by protein deficiencies or genetic mutations. These therapies can replace or supplement missing proteins, restoring normal cellular function:
- Acute Intermittent Porphyria (AIP): In AIP, a deficiency in porphobilinogen deaminase (PBGD) leads to a buildup of toxic precursors. Administering PBGD mRNA in animal models has shown rapid restoration of PBGD protein expression, offering a potential therapeutic approach for this rare disorder.
- Ornithine Transcarbamylase Deficiency: This urea cycle disorder leads to elevated ammonia levels and neurological damage. mRNA encoding ornithine transcarbamylase, when delivered via nanoparticles, has been shown to normalize plasma ammonia levels and improve survival in mice, offering hope for future treatments.
- Phenylketonuria (PKU): PKU is caused by mutations in the phenylalanine hydroxylase gene, leading to elevated levels of phenylalanine. mRNA-based therapies can deliver functional copies of this gene, reducing phenylalanine levels in animal models and providing a potential long-term solution for PKU.
Beyond infectious diseases, cancer, and rare diseases, mRNA technology is showing promise in a variety of therapeutic areas, including autoimmune diseases, metabolic disorders, and genetic conditions. By delivering functional proteins or correcting genetic mutations at the mRNA level, this approach could revolutionize the treatment of many challenging conditions.
Limitations and Challenges in mRNA Synthesis
Despite these advances, several challenges persist:
- RNase Degradation: mRNA molecules are inherently unstable and prone to degradation by ubiquitous ribonucleases. This necessitates stringent laboratory protocols and the use of RNase inhibitors.
- Yield and Purity: Low yields may result from suboptimal enzyme activity or template design. Double-stranded RNA contaminants, arising from self-complementary sequences, can elicit unwanted immune responses and reduce translational efficiency.
- Incomplete Processing: Inefficient capping or polyadenylation can lead to rapid degradation and poor translation.
- Limited Support for Multi-omics and AI-driven insights: Integrating RNA sequencing data with other biological data types, such as DNA or methylation, and applying advanced AI tools can be challenging for many laboratories.
- High Costs: RNA sequencing can be very expensive because of the cost of chemicals, sequencing machines, and data analysis. For many labs, these high costs make it hard to do large or repeated studies.
Given these persistent challenges, there is a clear need for a solution that streamlines and unifies the entire RNA sequencing process and makes high-quality, actionable data accessible and affordable to a wider range of researchers. This is where Biostate AI stands out as a partner.
How Biostate AI Simplifies mRNA Research from Sample to Insight
Biostate AI removes the technical burden of RNA sequencing, letting you focus on the biology, not the bioinformatics. From tiny, low-quality samples to complete transcriptome profiling, our platform handles everything with unbeatable speed, affordability, and precision.
Whether you’re exploring mRNA translation, protein expression, or disease biomarkers, Biostate AI gives you an all-in-one pipeline, powered by automation and enriched by AI.
Key Advantages:
- Complete Transcriptome Coverage: Capture mRNA, non-coding RNA, and degraded/low-input samples
- Lightning-Fast Turnaround: Results in as little as 1–3 weeks
- AI-Driven Insights: Ask scientific questions in plain English, no coding required
- Low Sample Requirement: Just 10ng RNA or 10µL blood
- Low RIN Compatibility: Works even with RIN as low as 2
- Multi-Omics Ready: Integrate RNA-Seq with WGS, methylation, or single-cell datasets
- End-to-End Automation: From raw reads to ready-to-publish results
- Secure, Scalable Platform: Built for both academic and clinical research
- Starting at $80 per sample: Industry-leading pricing for high-quality results
With these features, Biostate AI makes advanced RNA sequencing accessible, reliable, and cost-effective for every lab.
Final Words!
The field of mRNA synthesis continues to evolve rapidly, driven by technological innovations and expanding clinical applications. Therapeutic applications of mRNA technology extend beyond vaccines to include protein replacement therapy, cancer immunotherapy, and regenerative medicine.
However, the complexity of mRNA synthesis research demands sophisticated analytical approaches that can capture the full spectrum of transcriptional processes. Traditional methods often provide fragmented views of cellular activity, leaving critical gaps in understanding that can impact research outcomes and therapeutic development.
At Biostate AI, we recognize that researchers face increasing pressure to generate high-quality data while managing tight budgets and timelines. Our RNA sequencing services deliver unparalleled depth and accuracy, processing samples as small as 10 µL of blood, 10 ng of RNA, or single formalin-fixed paraffin-embedded (FFPE) slides.
Our pricing starts at $80 per sample, with turnaround times ranging from 1 to 3 weeks, offering exceptional value without compromising quality. Schedule Your Personalized Demo Today and discover how we can accelerate your mRNA synthesis research with cutting-edge technology and unparalleled support.
FAQ
What is the difference between mRNA synthesis and DNA replication?
mRNA synthesis (transcription) creates temporary RNA copies of genetic information, while DNA replication produces permanent DNA copies. DNA replication occurs during cell division to ensure each daughter cell receives complete genetic information. mRNA synthesis happens continuously as cells need specific proteins, creating short-lived messenger molecules that carry genetic instructions from nucleus to cytoplasm.
The key distinction lies in purpose and permanence. DNA replication maintains genetic integrity across generations, while mRNA synthesis enables dynamic protein production in response to cellular needs. Understanding this difference helps researchers appreciate why mRNA-based therapeutics offer unique advantages in clinical applications.
How does transcription differ from translation?
Transcription and translation are two distinct processes in gene expression. Transcription is the process by which an mRNA molecule is synthesized from a DNA template in the nucleus. It involves creating an RNA copy of the gene’s instructions. Translation, on the other hand, occurs in the cytoplasm, where the mRNA is used to assemble a chain of amino acids in the correct sequence to form a protein, with the help of ribosomes and tRNA.
What sample types work best for mRNA analysis?
Fresh or properly preserved samples provide the highest quality mRNA for analysis. Blood samples should be processed within hours of collection or preserved in RNA stabilization reagents. Tissue samples require immediate freezing or preservation to prevent RNA degradation.
Sample storage conditions significantly impact mRNA quality. Repeated freeze-thaw cycles should be avoided, and samples should be stored at -80°C for long-term preservation. Proper handling throughout the collection and storage process ensures reliable sequencing results.