The integrity and purity of RNA directly influence the reliability of downstream applications such as RT-qPCR, RNA-Seq, and transcriptome analysis.
Yet, RNA is highly susceptible to degradation by ubiquitous RNases, and even minor lapses in technique can compromise experimental outcomes. Many researchers encounter challenges in isolating high-quality RNA, including low yield, contamination with genomic DNA or proteins, and inconsistent results across replicates.
Understanding the principles behind RNA sample preparation is essential. This involves selecting appropriate lysis buffers, maintaining RNase-free conditions, and choosing isolation methods based on sample type and downstream needs. Each step, from tissue disruption to elution, requires careful optimization to maximize yield and preserve RNA integrity.
We will address these pain points by providing a detailed overview of RNA isolation strategies, practical troubleshooting tips, and evidence-based recommendations to help you achieve reproducible, high-quality RNA suitable for advanced molecular analysis.
- RNA sequencing offers deep insights into gene expression but requires high-quality RNA for reliable results.
- RNA isolation is a delicate process vulnerable to degradation by RNases and contamination by genomic DNA and proteins.
- Optimizing each step of RNA isolation, stabilization, lysis, and purification is crucial, especially for challenging samples like FFPE tissue or blood.
- Even minor errors in the RNA preparation process can lead to inconsistent or misleading results, making precision essential for success.
- Biostate AI provides a complete solution for RNA sequencing at budget-friendly costs, handling every step from sample collection to final insights.
What is RNA Sample Preparation and Isolation
RNA sample preparation and isolation refer to the set of laboratory techniques used to extract and purify ribonucleic acid (RNA) from biological materials such as cells, tissues, or environmental samples.
Why is this important?
This process is fundamental in molecular biology and biotechnology because the quality and integrity of isolated RNA directly impact the accuracy of downstream applications, including reverse transcription PCR (RT-PCR), RNA sequencing (RNA-Seq), and microarray analysis.
- High-quality RNA gives reliable results in gene expression studies.
- Contaminated or degraded RNA can lead to false or inconsistent data.
- Proper isolation and preparation let you compare samples and repeat experiments with confidence.
Clear RNA isolation and sample preparation help you avoid errors and get trustworthy data for your studies.
To prevent errors and ensure successful RNA isolation, it’s vital to optimize every step of the process, from stabilization to purification. So, it’s essential to understand the key steps of RNA isolation that can directly affect the outcome.
Key Steps of RNA Isolation Workflow
RNA is fragile and prone to degradation by RNases, which are found everywhere—on hands, surfaces, and in the air. Unlike DNA, RNA is temporary, making it more vulnerable. RNases are stable and remain active even after sterilization, so they start degrading RNA immediately upon cell lysis or tissue harvesting.
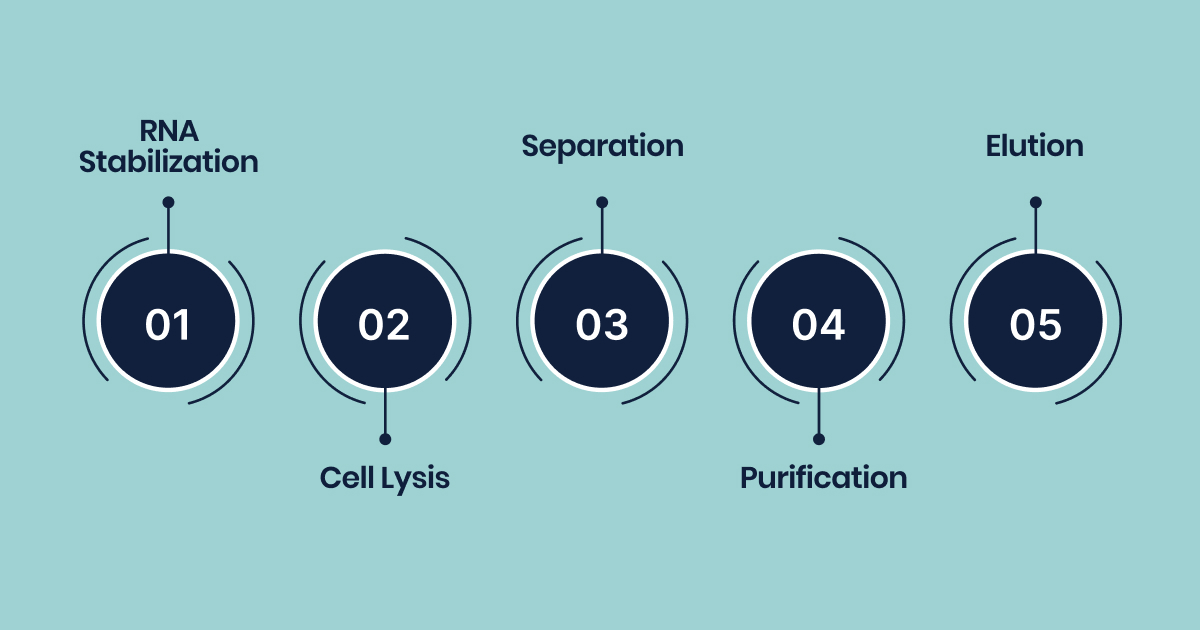
RNA isolation workflow involves critical stages, stabilization, lysis, separation, purification, and elution, each essential to preserve RNA integrity and maximize yield.
1. RNA Stabilization
The journey begins with RNA stabilization, a crucial initial step focused on preventing the immediate degradation of RNA. This often involves the rapid application of RNase inhibitors or other stabilizing agents to halt enzymatic activity.
2. Cell Lysis
Following stabilization, cell lysis, or cell disruption, is performed to break open cells or disrupt tissues, thereby releasing the cellular contents, including RNA, into solution. This can be achieved through various methods, including chemical approaches utilizing detergents or chaotropic agents, mechanical methods such as homogenization or bead milling, or enzymatic treatments that digest cell walls.
3. Separation
Once the cells are lysed, the subsequent step is separation, which aims to partition RNA from other biomolecules like DNA, proteins, and cellular debris. A classic method for this is phenol-chloroform extraction, which leverages differential solubility to isolate RNA into an aqueous phase.
4. Purification
After separation, purification steps are employed to remove residual contaminants, such as salts derived from extraction reagents. This typically involves additional wash steps, often with 70% ethanol or specific buffers.
5. Elution
Finally, the process concludes with elution or resuspension, where the purified RNA is recovered in a usable form, usually by dissolving it in a low salt buffer or nuclease-free water. It is worth noting that in some modern workflows, particularly column-based methods, the separation and purification steps can occur almost simultaneously through a series of binding, washing, and elution phases.
Protecting RNA throughout the isolation process is crucial, as small protocol errors can lead to degradation. The goal is to preserve the RNA accurately, reflecting the biological state without RNase interference.
This preparation lays the foundation for obtaining high-quality RNA, which is fundamental before we look at the specific methods researchers use to isolate RNA in various contexts.
Common RNA Isolation Methods
RNA isolation involves several methods, each with its own principles, advantages, and limitations. Choosing the right method is crucial, as it directly affects RNA quality, quantity, and the success of downstream applications.
Here are the key methods used in RNA isolation:
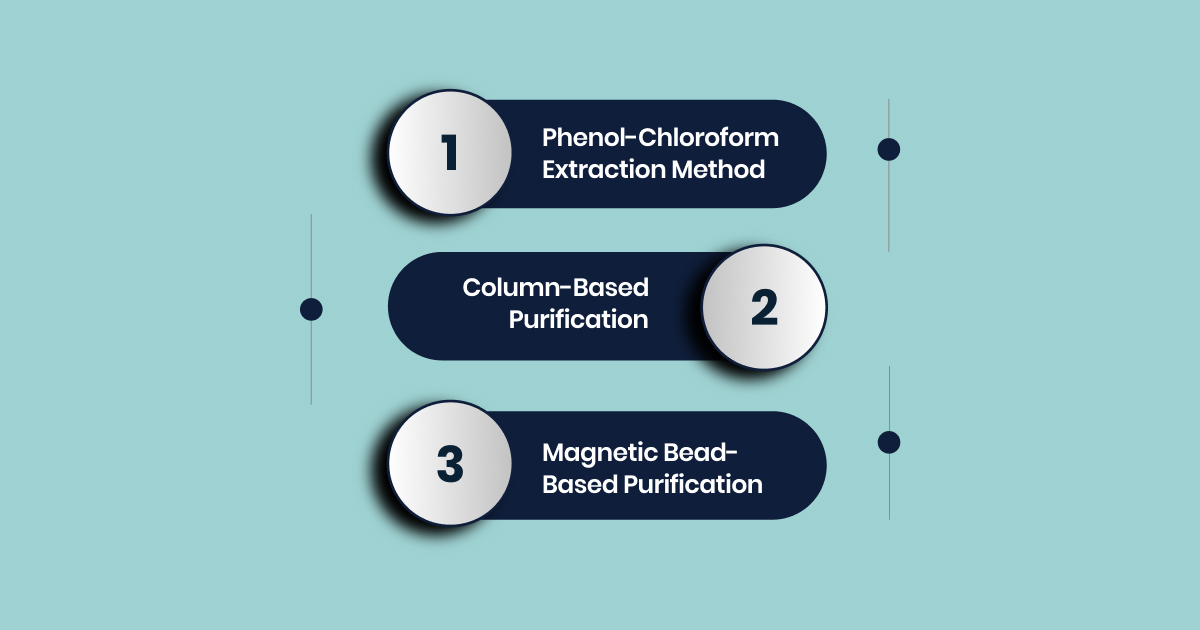
Phenol-Chloroform Extraction Method
Phenol-chloroform extraction is a traditional method that separates RNA from other cellular components based on their solubility in organic solvents. The process involves mixing the sample with a phenol-chloroform mixture, which denatures proteins and separates cellular components upon centrifugation.
RNA, being polar, stays in the aqueous phase, while DNA and lipids partition into the organic phase. Chloroform enhances the separation by increasing the density of the organic phase, ensuring better isolation of RNA and higher yields.
Key Characteristics:
- Effective in disrupting cells and denaturing proteins.
- High RNA yield, especially in tough or fibrous tissues, or those rich in proteins or secondary metabolites.
- Labor-intensive with multiple steps.
- Hazardous chemicals such as phenol and chloroform require careful handling.
- There is a risk of contamination due to the multi-step process.
- Stringent conditions are needed to prevent RNA degradation.
Column-Based Purification
Column-based RNA isolation methods have become widely adopted, particularly in commercial kits. These methods use spin columns with silica membranes or other affinity matrices.
Cells are lysed in a chaotropic buffer, which denatures proteins, including RNases, and helps RNA bind to the silica membrane. After applying the lysate, contaminants are washed away, and the RNA is eluted using a low-salt buffer or nuclease-free water.
DNase treatments are often incorporated to remove any residual genomic DNA.
Key Characteristics:
- Quick and efficient, offering high-purity RNA in less time.
- User-friendly with simple protocols suitable for high-throughput work.
- Low risk of contamination, as it avoids hazardous chemicals like phenol and chloroform.
- Risk of clogging if too much starting material is used, potentially affecting RNA yield.
- Limited capacity for binding large amounts of RNA, which can compromise both yield and purity.
- Commercial kits are available, making it convenient for routine lab work.
Magnetic Bead-Based Purification
Magnetic bead-based RNA isolation employs superparamagnetic particles or beads coated with substances like silica to bind RNA. After the biological sample is lysed and mixed with the beads, RNA binds to the surface of the beads.
An external magnetic field is used to pull the beads to the side of the tube, enabling easy removal of unbound contaminants. The purified RNA is then eluted from the beads.
Key Characteristics:
- Highly flexible with sample volume adjustments and high throughput capabilities.
- Minimal sample loss, providing high sensitivity even with low amounts of starting material, such as single cells.
- Easily automatable, making it ideal for high-throughput environments.
- No need for centrifugation equipment, beneficial for labs with limited resources.
- Sensitive, capable of isolating high-quality RNA from small cell populations.
There is no one-size-fits-all RNA isolation method. The best choice depends on three factors: the biological sample, the downstream application, and the laboratory’s practical constraints, including cost, safety, and throughput. Selecting the right method for your sample and application is crucial to avoid poor yield or failure.
However, even when the correct method is selected, there’s still the issue of how to handle RNA degradation, which brings us to the next crucial consideration: preservation.
How to Prevent RNA Degradation During Sample Preparation
RNA is highly susceptible to degradation, especially due to the omnipresence and stability of ribonucleases (RNases). Unlike most proteins, RNases are highly resistant to inactivation by typical sterilization methods, such as boiling or autoclaving, making them the primary challenge in RNA sample preparation.
Here are the multilayered approaches that need to be followed to prevent RNA degradation:
Establishing an RNase-Free Environment
To prevent RNA degradation, maintaining an RNase-free environment is crucial. This involves both good lab practices and effective decontamination protocols.
Lab Practices:
- Always wear gloves and change them frequently to avoid transferring RNases.
- Designate a specific RNA handling area, away from high-traffic zones.
- Clean work surfaces and equipment with RNase decontamination solutions (e.g., RNaseZap) or 100% ethanol.
Equipment and Plasticware:
- Use RNase-free disposable plasticware whenever possible.
- For reusable glassware, bake at 180°C for at least 4 hours to inactivate RNases.
- Plasticware that can’t be baked should be soaked in 0.1 M NaOH/1 mM EDTA or absolute ethanol with 1% SDS, rinsed with DEPC-treated water, and autoclaved.
- Decontaminate electrophoresis tanks with 1% SDS, rinse with ethanol, and soak in 3% H2O2 for 10 minutes, followed by DEPC-treated water.
Reagent Preparation and Storage:
- Use RNase-free reagents and store them separately from general lab reagents.
- Treat solutions with 0.1% DEPC or DMPC (except Tris buffers, which react with DEPC/DMPC) and autoclave to eliminate RNases.
- Always reserve a dedicated bottle of Tris for RNA work and treat all glassware and water with DEPC/DMPC.
Sample Handling and Stabilization Techniques
RNA degradation starts as soon as a sample is collected. To protect RNA, follow these best practices:
- Immediate Processing or Freezing: Flash-freeze samples in liquid nitrogen and store at -70°C or -80°C to prevent endogenous nuclease activity.
- RNA Stabilization Solutions: Use reagents like RNAlater or Tempus Blood RNA Tubes, which prevent RNase activity and stabilize RNA in the sample.
- Tissue Samples: Ensure tissue pieces are small enough to freeze quickly, reducing the risk of degradation during freezing.
- Storage: Once extracted, store RNA in single-use aliquots at -80°C to prevent degradation from repeated freeze-thaw cycles.
The Role of RNase Inhibitors
RNase inhibitors, such as Protector RNase Inhibitor or RNasin, provide essential protection during RNA extraction and downstream applications.
- These inhibitors bind to and inactivate RNases, offering added security during sensitive stages of RNA handling.
- Many inhibitors work over a broad temperature range, making them effective during processes like reverse transcription.
Increasing the concentration of RNase inhibitors can offer enhanced protection for particularly difficult samples.
But even with careful handling, some samples, such as those with low RNA integrity, present additional challenges. This brings the next topic of discuss which is how to optimize RNA yield for low RNA integrity samples.
Sample-Specific Strategies for Optimizing RNA Yield
Achieving a high yield of high-quality RNA is a primary objective in sample preparation. Protocols should be tailored to the specific sample type to maximize RNA yield and quality.
Here’s a breakdown of different sample types and considerations for each:
Plant Samples (Secondary Metabolites)
Plant tissues contain secondary metabolites like phenolics and quinones, which can interfere with RNA isolation. These compounds can bind to RNA, inhibit enzymatic reactions, or hinder RNA resuspension. To address this:
- Effective methods: Phenol-chloroform extraction and TRIzol remain gold standards due to their ability to denature proteins and separate secondary metabolites.
- Modified protocols: Guanidinium-free systems or specialized buffers can prevent phenolic oxidation and improve RNA yield and purity.
Animal Tissues (Fibrous, Low-Yield)
RNA content varies across animal tissues. Organs like the liver yield higher RNA, while tissues like muscle and brain tend to give lower yields. Key considerations include:
- Immediate processing: Flash-freeze or process tissues quickly to prevent degradation by nucleases.
- RNA stabilization: Use RNAlater for longer-term preservation before extraction.
- Mechanical disruption: For fibrous tissues, use methods like rotor-stator homogenizers or liquid nitrogen to enhance cell lysis.
- Proteinase K optimization: Adjust concentration and incubation time for specific tissue types to improve RNA yield.
Blood Samples (Globin Removal)
Blood samples are challenging due to the high abundance of globin mRNA, which can dilute target mRNA. To manage this:
- Specialized collection tubes: Tempus or PAXgene tubes stabilize RNA and prevent degradation.
- Globin depletion methods: Biotinylated DNA oligos or peptide nucleic acid oligos remove over 95% of globin mRNA, enhancing detection.
- Yield trade-offs: Globin depletion can reduce RNA yield and affect RNA quality (e.g., RNA Integrity Number), requiring a balance based on experimental needs.
Cell Culture Samples (Low Cell Numbers)
For low-input samples, such as sorted cells or laser-captured microdissection (LCM) samples, sensitive RNA isolation is crucial. Key factors to consider:
- Sensitive kits: Use specialized kits like Norgen’s Total RNA Purification Micro Kit for efficient RNA extraction from small samples.
- Denaturing agent concentration: Ensure lysis buffer concentration is adequate to prevent RNase activity and degradation.
- Complete lysis: For difficult-to-lyse cells (e.g., lymphocytes), use concentrated reagents like TRIzol LS for effective disruption.
- Small elution volumes: Kits designed to elute RNA in small volumes (e.g., 20 µL) are ideal for high-sensitivity downstream applications.
However, regardless of the sample type, several overarching challenges in RNA isolation persist, which is why addressing these issues upfront is critical for successful research.
Challenges in RNA Isolation and Sample Preparation
The quality and integrity of isolated RNA directly impact downstream applications such as gene expression analysis, sequencing, and diagnostics. However, these steps present several technical challenges that can compromise results. Understanding these challenges helps researchers optimize protocols and improve data reliability.
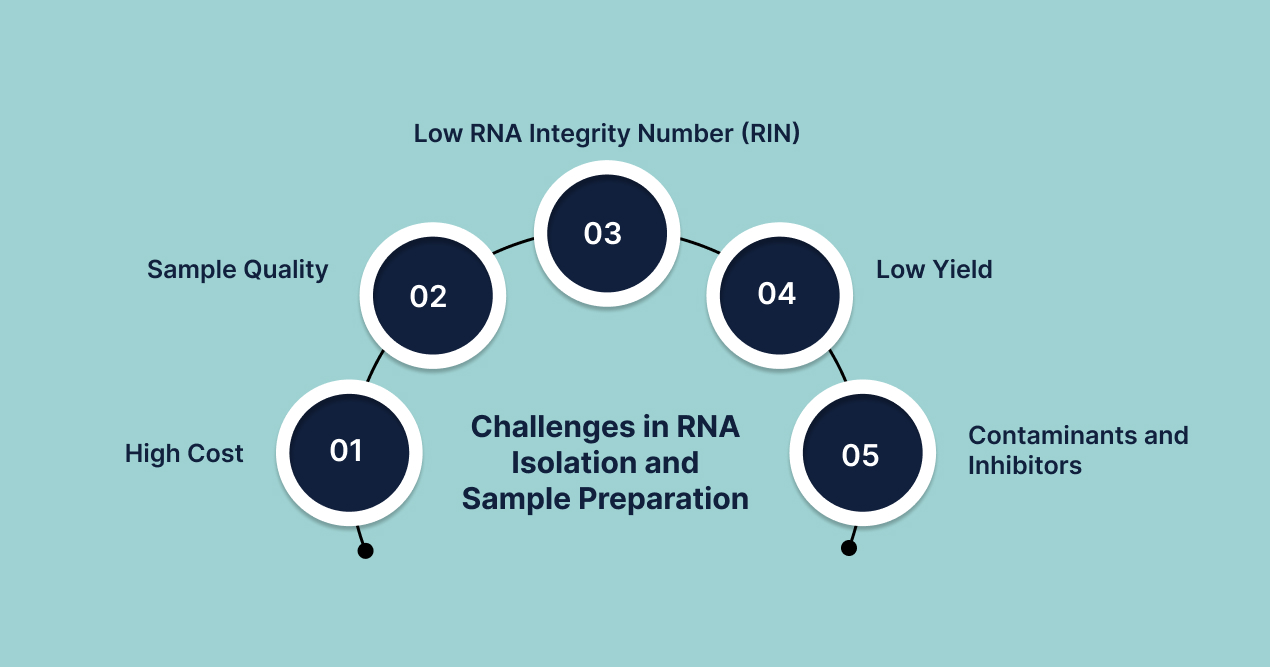
1. High Cost
Specialized reagents, consumables, and equipment for RNA extraction are expensive. Automated platforms and high-throughput kits further increase costs, especially for large-scale or clinical studies. This financial barrier can limit the number of samples processed and the feasibility of replicates, affecting experimental robustness.
2. Sample Quality
Sample quality is a major determinant of RNA integrity. Factors such as improper collection, delayed processing, or suboptimal storage conditions can lead to RNA degradation before extraction begins. Clinical and archived samples, such as FFPE tissues, are particularly prone to degradation and chemical modifications that complicate isolation and analysis.
3. Low RNA Integrity Number (RIN)
RIN is a standard metric for RNA quality. Low RIN values indicate degraded RNA, which can result from RNase contamination, harsh extraction conditions, or poor sample handling. Low RIN indicates RNA degradation, which affects the accuracy of applications like RNA-seq and qPCR by reducing transcript representation and increasing variability.
4. Low Yield
Many biological samples, such as fine needle aspirates or single cells, provide minimal starting material. Inefficient lysis, loss during purification, or inadequate protocol optimization can further reduce RNA yield. Low yield limits the ability to perform comprehensive analysis and may require amplification steps that introduce bias.
5. Contaminants and Inhibitors
Co-purification of DNA, proteins, phenol, or other inhibitors is common, especially with certain extraction methods. These contaminants can inhibit enzymatic reactions in downstream workflows and skew quantification results. Rigorous protocol optimization and, if necessary, post-extraction clean-up steps are essential to minimize these issues.
Addressing these challenges requires careful sample handling, protocol optimization, and investment in quality reagents and equipment. Consistent quality control at every step is essential for robust and reproducible RNA-based research. This is where Biostate AI can be just the solution for you.
How Biostate AI Can Become an Effective Tool for RNA-Sequencing
Biostate AI addresses the most persistent technical challenges in RNA-Seq with a comprehensive, integrated platform designed for research scholars and scientists. Here’s how it effectively overcomes issues such as high cost, variable sample quality, and low RNA integrity:
- Affordable RNA-Seq at Scale: Biostate AI offers RNA sequencing starting at $80 per sample. Our automation and proprietary technologies reduce costs by up to 70% compared to traditional providers.
- Accepts Versatile Samples: We accept a wide range of sample types, including blood, tissue, cell cultures, and FFPE slides. Protocols are optimized for both fresh and archived samples.
- Low RIN Compatibility: Biostate AI can process RNA samples with RIN values as low as 2.
- Minimal Sample Requirement: The system works with very small input amounts: as little as 10 µL blood, 10 ng RNA, or a single FFPE slide.
- End-to-End Automation and AI-Driven Analysis: Our OmicsWeb platform provides automated, AI-powered analytics. Automated pipelines ensure consistency, reproducibility, and a rapid turnaround of 1–3 weeks.
Our all-in-one platform eliminates fragmented workflows and democratizes access to advanced bioinformatics. Researchers can focus on scientific discovery while Biostate AI ensures data quality, scalability, and actionable results.
Final Words!
The RNA isolation process is delicate and requires precise handling at every step, from stabilization to purification. Any mistake can compromise data integrity, especially with difficult samples like FFPE tissue, blood, or low-input samples. Proper optimization is crucial to ensure reliable, high-quality RNA for accurate results.
This is where Biostate AI comes in. We offer dedicated protocols for a wide variety of sample types, including hard-to-process samples with low RNA integrity, and their fast turnaround time ensures researchers stay on track with their studies.
With RNA sequencing starting as low as $80 per sample, we make high-quality RNA sequencing accessible without sacrificing accuracy or speed.
If you are looking for a reliable, cost-effective solution to streamline your RNA sequencing process, Biostate AI is your ideal partner. Get in touch with us today to know how we can help take your research to the next level.
FAQs
1. What are the main differences between column-based and reagent-based RNA extraction methods?
Column-based methods, such as silica spin columns, use selective binding properties to purify RNA, resulting in high purity and speed. These kits often include on-column DNase treatment to remove DNA contamination. Reagent-based methods, like TRIzol or phenol-chloroform extraction, rely on organic solvents to separate RNA from DNA and proteins. While reagent-based methods can yield high quantities, they require careful handling of hazardous chemicals and additional cleanup steps for purity.
2. What factors should be considered when selecting an RNA isolation method for blood samples?
Considerations include the initial RNA yield (which is typically low in blood), the presence of inhibitors (such as heme), and the need for rapid processing to prevent degradation. Using RNase-free consumables and stabilization reagents immediately after collection is crucial. The method should also be compatible with downstream applications and allow for efficient removal of genomic DNA and proteins.
3. What are common quality control steps after RNA isolation?
Assess RNA concentration and purity using spectrophotometry (A260/A280 and A260/A230 ratios) or fluorometric quantitation (e.g., Qubit). Evaluate RNA integrity using capillary electrophoresis (e.g., Agilent Bioanalyzer) or agarose gel electrophoresis. Samples with a high RNA Integrity Number (RIN, typically above 7) are preferred for most downstream applications.
4. What are the safety considerations when using chemical reagents for RNA extraction?
Chemical reagents like phenol and chloroform are toxic and require appropriate personal protective equipment (PPE), fume hoods, and waste disposal procedures. Always follow manufacturer safety guidelines and institutional protocols to minimize exposure and environmental impact.
