Did you know? The global market for single-cell genome sequencing is growing rapidly, expanding from $4.31 billion in 2024, and is expected to reach $16.64 billion by 2033.
Why this explosive growth? Single-cell genome sequencing captures the unique genetic profile of each cell, providing unmatched detail and uncovering cellular diversity missed by traditional bulk sequencing methods that average data from millions of cells.
This technology is especially useful in cancer research, where cellular differences drive disease progression and resistance to treatment. It also plays a key role in discovering biomarkers, stratifying patients, and developing personalized therapies.
In this article, we’ll dive into the innovative techniques behind single-cell sequencing and explore its impact across fields like cancer, neuroscience, developmental biology, and immunology.
Key Takeaways
- Single-cell genome sequencing enables detailed analysis of individual cells, uncovering cellular diversity and disease mechanisms missed by traditional bulk sequencing methods.
- Techniques like droplet-based microfluidics and fluorescence-activated cell sorting (FACS) have advanced the field, enabling high-throughput analysis and improved sensitivity for rare cell populations.
- Applications span across cancer research, neuroscience, developmental biology, and immunology, providing insights into tumor heterogeneity, neurodegenerative diseases, and immune responses.
- Despite its promise, challenges such as cost, sensitivity, technical artifacts, and data analysis complexity persist, limiting its widespread adoption.
- Biostate AI offers a solution, streamlining the process with lower sample requirements, faster timelines, and cost-effective sequencing, making single-cell analysis more accessible for research and drug development.
What is Single-Cell Genome Sequencing
Single-cell genome sequencing represents a revolutionary technological approach that examines nucleic acid sequence information from individual cells at a time, revealing the unique genetic makeup and molecular characteristics of each individual cell.
The methodology encompasses several distinct but related approaches. For example,
- Single-cell RNA sequencing (scRNA-seq) captures the complete transcriptomic landscape of individual cells, revealing which genes are actively expressed at specific timepoints or cellular states.
- Single-cell DNA sequencing (scDNA-seq) analyzes genomic variations, mutations, and copy number alterations within individual cells, particularly valuable for understanding clonal evolution in cancer.
- Single-cell multi-omics approaches combine multiple molecular layers, including genomics, transcriptomics, epigenomics, and proteomics, within the same cell to provide comprehensive molecular portraits.
The technology behind single-cell sequencing uses advanced molecular barcoding. Each cell is given a unique barcode during library preparation. This barcode stays attached to all molecules from that cell throughout the sequencing.
When the sequencing data is mixed, the barcode allows researchers to trace each read back to its original cell. This process reconstructs individual cell profiles from the pooled data, giving a clear view of cellular details.
Key Technical Parameters:
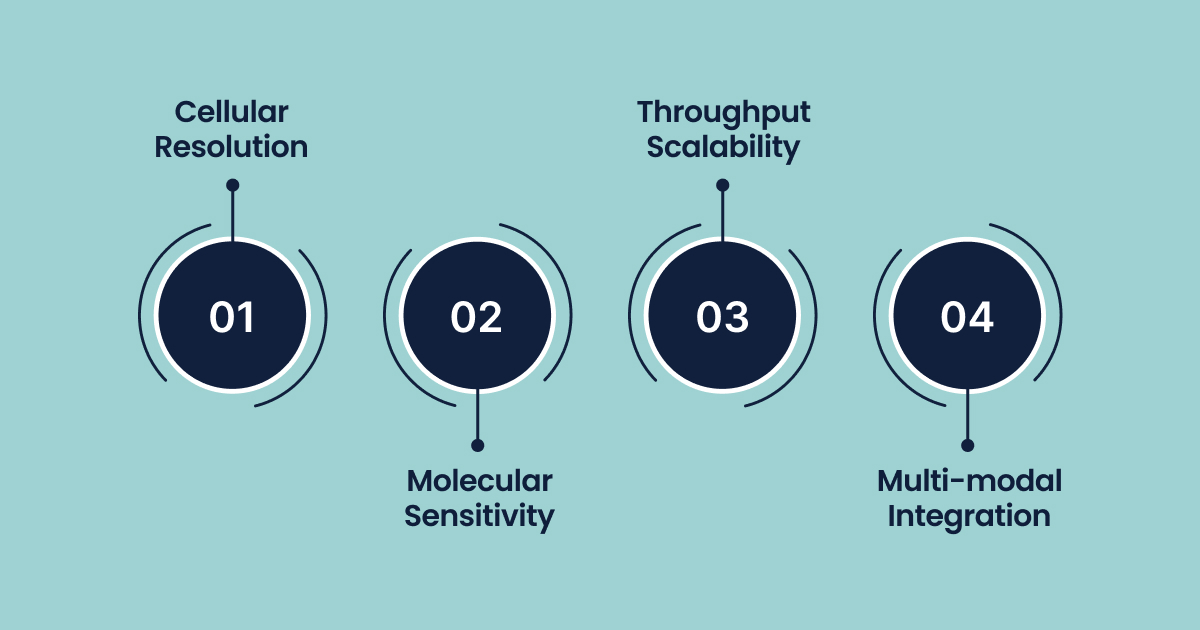
- Cellular Resolution: Analysis of 10² to 10⁶ individual cells per experiment, depending on the platform and research objectives
- Molecular Sensitivity: Detection of 10³ to 10⁴ genes per cell in scRNA-seq applications, with ongoing improvements in capture efficiency
- Throughput Scalability: Modern platforms process 10⁴ to 10⁶ cells per experimental run, enabling population-scale studies
- Multi-modal Integration: Simultaneous measurement of genomic, transcriptomic, and epigenomic features within identical cells
In the next section, we will discuss different techniques of single-cell genome sequencing.
Techniques of Single-Cell Genome Sequencing
Single-cell genome sequencing encompasses multiple sophisticated methodologies designed to extract, amplify, and analyze genetic material from individual cells. These techniques have evolved to address the challenges of working with femtogram quantities of DNA and RNA while maintaining genomic integrity and minimizing artifacts.
Droplet-Based Microfluidics
Droplet Microfluidics Platforms represent the current gold standard for high-throughput single-cell analysis.
- The process leverages the Poisson distribution for cell encapsulation, where typically only 10-15% of droplets contain single cells under optimal conditions, while most remain empty.
- The monodispersity of these picoliter-volume droplets (1 pL to 10 nL) enables quantitative control of solute concentrations while providing isolated compartments that maintain high signal-to-noise ratios for cellular assays
- Recent innovations enable processing of over 50,000 single cells per experiment, with companies like 10x Genomics launching GEM-X technology that doubles gene detection sensitivity while reducing cost per cell by more than half.
- Roche uses it to analyze patient tumor samples across multiple cancer types simultaneously.
- Novartis employs it to track immune responses during clinical trials. The ability to process large patient cohorts efficiently makes population-scale studies feasible.
However, this technique isn’t perfect. Sensitivity remains limited compared to plate-based methods. But for high-throughput screening and population studies, droplet microfluidics sets the standard.
Fluorescence-Activated Cell Sorting (FACS) Mechanism
FACS operates through a sophisticated integration of fluidic, optical, and electrostatic systems.
- Cells flow through a nozzle vibrated at an optimal frequency to produce droplets at fixed distances.
- When cells pass through laser interrogation points, forward-scattered light (FSC) indicates cell size while side-scattered light (SSC) reflects granularity.
- Fluorescence detection from labeled antibodies enables the identification of specific cell populations.
The critical innovation lies in the precise timing: as droplets form, electrical charges are applied based on real-time fluorescence measurements, allowing electrostatic deflection of desired cells into collection containers.
Multiple Displacement Amplification (MDA) and Advanced Amplification Methods
MDA utilizes phi29 DNA polymerase, a highly processive enzyme with extraordinary strand displacement capability.
- The phi29 polymerase contains a specific subdomain called Terminal Protein Region 2 (TPR2) that forms DNA-binding tori, enabling the enzyme to topologically encircle both upstream duplex and downstream template DNA.
- Recent developments include:
- MALBAC (Multiple Annealing and Looping-Based Amplification Cycles)
- LIANTI (Linear Amplification via Transposon Insertion)
- META-CS technologies
These methods achieve genome coverage exceeding 93% at 30× sequencing depth while minimizing amplification bias that historically compromised single-cell genomic analysis.
Third-Generation Sequencing (TGS)
The integration of TGS represents an emerging frontier combining single-cell approaches with long-read sequencing technologies.
- They can detect structural variations, alternative splicing events, and complex genomic rearrangements invisible to short-read sequencing.
- Platforms include:
- SCAN-seq
- ScISOr-Seq
- RAGE-seq
These technological advances position single-cell genome sequencing as an indispensable tool for pharmaceutical and biotechnology companies pursuing precision medicine strategies.
Single-cell sequencing offers a range of technical approaches to meet the diverse needs of pharmaceutical research. Some applications require high-throughput screening of large cell populations, while others focus on the precise analysis of rare cell types. Understanding these technical capabilities is key to unlocking breakthrough applications in various therapeutic areas.
Applications of Single-Cell Genome Sequencing
Single-cell genome sequencing provides unprecedented insights into disease mechanisms, therapeutic targets, and treatment responses.
Cancer Research
Cancer represents the largest application segment for single-cell technologies, accounting for 35.75% of the global market. This is driven by the unique ability of single-cell genome sequencing to dissect clonal evolution and identify rare cancer stem cell populations.
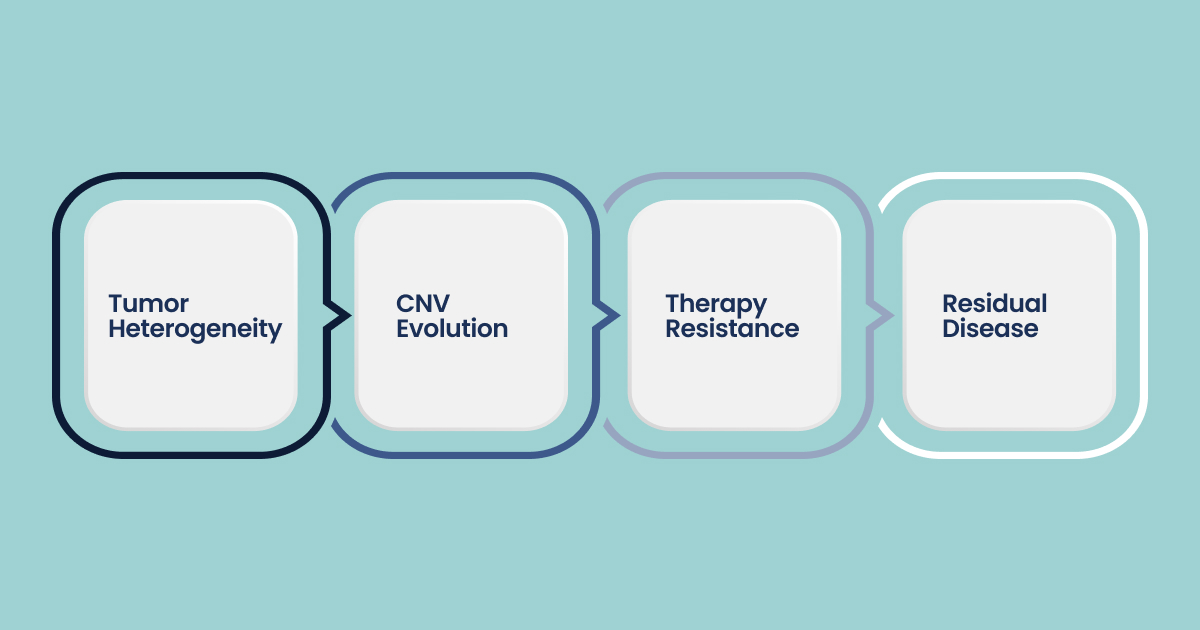
1. Resolving Intra-Tumor Heterogeneity
Tumors rarely involve a single homogeneous cell population. scDNA-seq can directly count and characterize subclones without deconvolution assumptions.
| Cancer Type | Key Insight |
| Metastatic colorectal | Demonstrated late dissemination via monoclonal or polyclonal seeding, tracing primary and liver metastasis cells genome-wide. |
| Colon | Found convergent evolution of CNVs between primary tumor cells and circulating tumor cells (CTCs), suggesting selection toward metastatic competency. |
| Breast | Identified “stem” mutation trunk followed by rapid sweep and neutral diversification, refining evolutionary timing models. |
These studies underscore how scDNA-seq deciphers clonal architecture that guides prognosis and personalized therapy decisions.
2. Copy-Number Evolution and Punctuated Dynamics
Hepatocellular carcinoma scDNA-seq revealed a dual-phase CNA model with an initial punctuated burst followed by gradual accumulation; longer gradual phases predicted worse survival.
Lung cancers showed parallel clonal copy-number catastrophes distinguished by polyploidization events. Such cell-level granularity redefines how CNVs drive oncogenesis.
3. Tracking Therapy Resistance in Real Time
Longitudinal scDNA-seq during FLT3 inhibitor treatment pinpointed the emergence of FLT3-D835Y subclones resistant to sorafenib, while sensitive clones were eradicated.
Similarly, IDH2-inhibitor exposure selected RAS-mutant and IDH1-mutant branches. Clone-by-clone pharmacodynamic read-outs can steer adaptive clinical interventions.
4. Minimal Residual Disease and Liquid Biopsy Integration
High-throughput droplet scDNA-seq platforms now process millions of cells, supporting detection of rare residual leukemic clones at parts-per-million sensitivity—a leap beyond bulk sequencing.
Coupling scDNA-seq with circulating tumor DNA allows cross-validation of emerging resistant lineages
Biomarker Discovery
Biomarker discovery benefits significantly from single-cell approaches.
- This identifies cell-type-specific markers invisible to bulk sequencing.
- Studies show that single-cell analysis can identify biomarkers predictive of treatment response, including specific T cell populations associated with positive outcomes to anti-PD-1 therapy.
- These discoveries enable precision patient stratification and companion diagnostic development.
Applications in Neuroscience
The human brain contains hundreds of distinct cell types, each potentially contributing to disease pathogenesis through unique molecular mechanisms.
1. Somatic Mosaicism in the Brain
scDNA-seq shattered the dogma of a genetically uniform brain. Key observations include:
| Finding | Evidence | Impact |
| 13–41% of human frontal cortex neurons harbor ≥1 megabase CNV. | Single-neuron WGA and low-coverage sequencing. | Suggests widespread copy-number mosaicism influencing neuronal diversity. |
| Neurons accumulate ~800–2,000 SNVs linearly with age (“genosenium”). | 161 single neurons aged 4 months–82 years. | Links to age-related cognitive decline and genome-instability diseases. |
| Brain Somatic Mosaicism Network generated >400 TB multi-omic data on neuropsychiatric disorders. | Consortium scale. | Provides reference brain genomes to benchmark mutation discovery. |
2. Neurodevelopment and Lineage Reconstruction
Retrospective scDNA-seq using spontaneous SNVs reconstructs clonal dispersion patterns, revealing early progenitor mixing across cortical areas. Prospective CRISPR barcode lineage tracing (e.g., TracerSeq) charted >92,000 zebrafish embryonic brain cells, linking spatial patterning to lineage.
3. Neurodegeneration and DNA Damage
Neurons from Cockayne syndrome and xeroderma pigmentosum show 2.3- to 2.5-fold excess SNVs compared with age-matched controls, correlating with defective DNA-repair genes. Single-cell long-read sequencing further uncovered active LINE-1 retrotransposition in aged human brains.
4. Epigenome and Multi-Modal Maps
Combined TACIT and CoTACIT methods achieve genome-wide histone-mark profiling plus lineage barcodes from mouse zygote to blastocyst, exposing chromatin lineage priming. Integrative single-cell atlases now overlay transcription, DNA methylation, and CNVs across the human lifespan.
Applications in Developmental Biology
Single-cell genome sequencing enables the capture of cellular transitions and lineage relationships, which are essential for understanding tissue formation and organ development.
1. Preimplantation Genetic Diagnosis and Chromosomal Mosaicism
- scDNA-seq of trophectoderm and inner cell mass in 55 blastocysts showed mosaic aneuploidy in ≥82% embryos, often affecting <20% cells—below bulk preimplantation genetic testing detection limits.
- Genome recovery strategies for β-thalassemia and COL4A1 mutations leveraged single-blastomere WGA to phase full embryonic genomes, enabling comprehensive PGD.
2. Lineage-Tracing at Embryo Scale
- Dynamic barcoding (CRISPR or transposon) plus scDNA-seq reconstructs embryo-wide cell-state landscapes with branching or convergent routes, as in zebrafish and mouse gastrulation.
- Recent SMALT adaptation in zebrafish recorded a median 14 substitution barcodes per cell, achieving single-cell resolution lineage of original versus regenerated fin tissue.
3. Mutation Rates and Chromatin Context
Early mouse neurogenesis exhibits sub-megabase CNVs arising during cortical development, validated by transposase-based single-cell whole-genome sequencing and machine-learning CNV filters. Such developmental CNVs may create lasting neuronal circuits or disease predisposition.
4. Human Embryo Epigenetic Reprogramming
Genome-coverage single-cell histone modification profiles reveal heterogeneity as early as the 2-cell stage, back-tracking ICM and TE specification to the 8-cell embryo and identifying TFs driving lineage choice.
Applications in Immunology
Single-cell genome sequencing revolutionizes immunology research by revealing the extraordinary diversity of immune cell populations and their responses to pathogens, vaccines, and immunotherapies.
1. V(D)J Repertoire Deconvolution
Single-cell DNA (or linked cDNA) sequencing captures complete T-cell receptor (TCR) and B-cell receptor (BCR) rearrangements, allowing direct pairing of α/β or heavy/light chains. Unique molecular identifiers and real-time PCR (rhPCR) reduce amplification bias, enabling quantitative clonotype counting.
2. Clonal Selection and Expansion
- scDNA-seq revealed oligoclonal γδ T-cell expansions in healthy donors, mapping paired TRDV/TRDJ usage at nucleotide resolution.
- In aged mouse CNS, clonally expanded T and B lymphocytes with restricted repertoires populate brain parenchyma, implicating adaptive immunity in neuroinflammation.
3. Somatic Hypermutation Dynamics
By merging scRNA-seq with BCR genotyping, researchers quantified active somatic hypermutation (SHM) events in follicular lymphoma cells hours after occurrence, linking AID expression to intratumoral diversity. Mechanistic reviews detail five distinct DNA-repair pathways shaping SHM patterns and A/T versus C/G biases.
4. Immune Response Trajectories and Sepsis
scRNA-seq plus immune-repertoire analysis tracked BCR diversity collapse and impaired antigen presentation within hours of experimental sepsis, highlighting single-cell approaches to systemic immune dysregulation.
5. Multi-Omic Interrogation of Signaling States
Paired single-cell chromatin accessibility, transcriptome, and TCR clonotype data (e.g., scATOMIC) classify tumor-infiltrating lymphocytes, predict exhaustion states, and cross-reference neoantigen reactivity.
These applications demonstrate single-cell sequencing’s maturation from research curiosity to an essential pharmaceutical tool. The technology provides actionable insights that translate directly into improved therapeutic strategies.
However, realizing this potential requires overcoming significant technical and practical challenges that continue to shape the field’s evolution.
Challenges and Limitations of Single-Cell Genome Sequencing
Despite revolutionary advances, single-cell genome sequencing faces significant obstacles that pharmaceutical researchers must navigate carefully. Understanding these limitations ensures appropriate experimental design and realistic expectations for research outcomes.
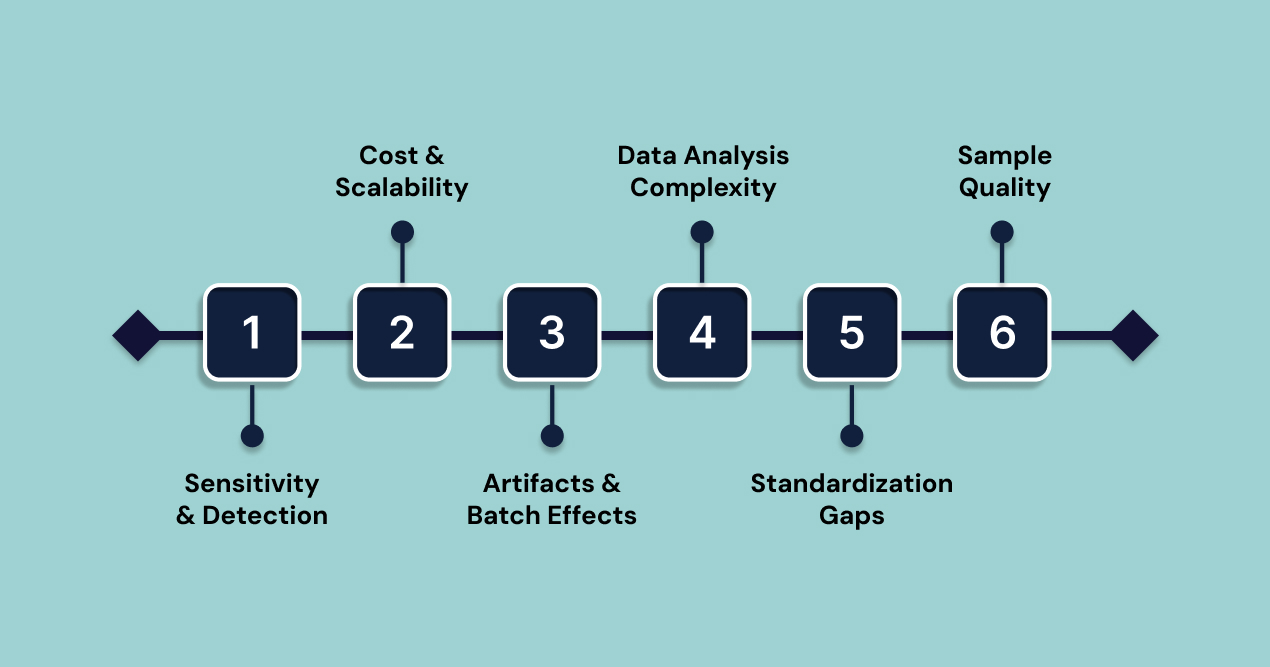
1. Sensitivity and Detection Limits
Current technologies capture only 10-20% of mRNA molecules within individual cells. This limited sensitivity means low-abundance transcripts, often the most biologically interesting, frequently go undetected. Critical regulatory molecules, rare splice variants, and early response genes may be missed entirely. Pharmaceutical researchers must account for these blind spots when interpreting results.
2. Cost and Scalability Barriers
Processing costs, while decreasing, still limit study scope. Large-scale population studies or comprehensive drug screening experiments require substantial budgets. Many pharmaceutical companies struggle to justify the expense of exploratory research. Academic-industry partnerships often emerge as cost-sharing solutions, but intellectual property complexities can limit collaboration.
3. Technical Artifacts and Batch Effects
Sample preparation procedures introduce artificial signals that can overwhelm biological differences. Cell dissociation protocols alter gene expression patterns. Different experimental batches show systematic variations unrelated to biology. These technical confounders require sophisticated computational corrections that may inadvertently remove real biological signals.
4. Data Analysis Complexity
Single-cell datasets generate terabytes of high-dimensional data requiring specialized computational infrastructure and expertise. Most pharmaceutical companies lack dedicated bioinformatics teams for single-cell analysis. Outsourcing analysis introduces delays and communication barriers that slow research timelines.
5. Standardization Gaps
The field lacks universally accepted protocols and quality metrics. Different platforms generate incompatible data formats. Cross-study comparisons become challenging or impossible. Regulatory agencies struggle to evaluate single-cell data without standardized guidelines, potentially complicating drug approval processes.
6. Sample Quality Requirements
Many clinical samples arrive degraded, and traditional single-cell protocols require high-quality, freshly isolated cells, limiting the types of patient samples that can be analyzed.
Addressing these challenges requires more accessible, reliable, and cost-effective technological solutions. To overcome these limitations, platforms must combine technical innovation with practical considerations for real-world research environments.
How Biostate AI Can Be a Breakthrough Addition to Your RNA-Sequencing Research
Current single-cell genomics workflows create significant barriers for pharmaceutical research teams. Biostate AI eliminates these traditional barriers through an integrated platform that combines advanced sample processing with AI-enhanced analytical capabilities.
Technical capabilities:
- Ultra-Low Sample Requirements: Analyze samples as small as 10µL blood, 10ng RNA, or single FFPE slides—enabling analysis of precious clinical specimens and rare cell populations that traditional methods cannot process
- Degraded Sample Compatibility: Process RNA samples with RIN values as low as 2, compared to industry-standard requirements of ≥5, unlocking analysis of challenging clinical samples previously considered unusable
- Accelerated Timelines: Deliver comprehensive results within 1-3 weeks versus months required by traditional workflows, enabling rapid experimental iteration and faster research progress
- Cost-Effective Processing: High-quality sequencing starting at $80 per sample makes large-scale studies financially accessible while maintaining pharmaceutical-grade quality standards
- AI-Powered Analytics: OmicsWeb AI platform provides intuitive data analysis through natural language querying, eliminating computational bottlenecks that delay insight generation
- Complete Transcriptomic Coverage: Total RNA sequencing captures both coding and non-coding transcripts, providing comprehensive molecular profiles essential for understanding complex cellular states
Our integrated approach transforms single-cell RNA sequencing from a specialized technique requiring extensive infrastructure into an accessible tool without compromising scientific quality or regulatory compliance.
Final Word
Single-cell genome sequencing reveals cellular complexity missed by bulk methods, identifying new therapeutic targets and mapping immune responses critical for therapy development. These insights are now crucial for modern biomedical research.
Companies using single-cell genomics gain a competitive edge by improving target identification, patient stratification, and reducing late-stage clinical failures. This leads to faster development and higher success rates for new therapies.
Biostate AI’s RNA sequencing platform addresses the challenges of high costs, complex sample needs, and technical expertise. By making single-cell analysis more accessible, we enable researchers to unlock breakthrough insights, regardless of their budget or resources.
The future of therapy development lies in precision at the cellular level. As single-cell technology moves towards clinical use, early adopters will gain lasting advantages in the evolving pharmaceutical landscape.
Reach out to us to learn how our RNA sequencing solutions can accelerate your research and therapeutic discoveries.
FAQs
Q: Can single-cell genome sequencing be integrated with existing clinical NGS workflows, or does it require entirely new infrastructure?
Yes, integration is increasingly feasible. Many modern single-cell sequencing platforms are designed to leverage existing next-generation sequencing (NGS) instruments and computational infrastructure. Full-scale adoption requires automation for single-cell isolation and bioinformatic pipelines, along with initial investment and cross-team training to handle increased data complexity and sample volume.
Q: How does single-cell genome sequencing support regulatory submissions and compliance in drug development?
Single-cell data can strengthen regulatory filings by providing high-resolution evidence of target modulation, patient stratification, and safety profiling that bulk approaches may miss. Regulatory bodies increasingly recognize single-cell data in IND and NDA dossiers, but recommend standardized reporting, benchmarking against validated controls, and explicit documentation of computational methodologies for reproducibility.
Q: What are the most underestimated pitfalls in designing a single-cell genome sequencing experiment for clinical or translational research?
Sampling bias and cell viability loss during dissociation are frequently overlooked, leading to underrepresentation of certain cell types or altered gene expression profiles. Inadequate planning for batch effects, cross-contamination, or low capture efficiency can also profoundly affect data interpretation. Upstream pilot studies and robust QC thresholds are critical for de-risking large projects.
Q: How does single-cell sequencing data management impact IP (Intellectual Property) and data security in pharma collaborations?
Single-cell datasets are uniquely granular and may contain proprietary clinical or preclinical insights ripe for IP generation and competitive edge. Secure, auditable data management systems are essential—not just for compliance but to protect against data leaks, establish clear IP provenance for patents, and facilitate seamless multi-site collaborations while fulfilling data-sharing agreements.
